Instruments

Instruments
Instrumentation and services are available to all researchers inside and outside of the university.
Researchers from the Northwestern University and the University of Chicago are provided internal rates as all UIC users. All other users can access services but at an external academic or external non-academic rate, which is determined using subsidy and market rates.
All users are responsible for their own laboratory safety training to be current during each use of the core facilities.
CYTEK Aurora
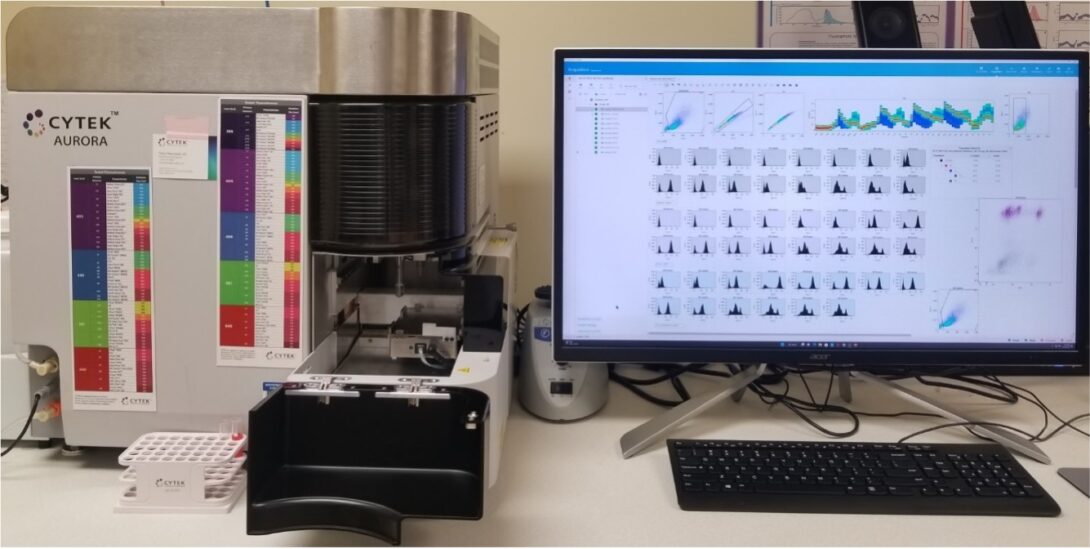
The CYTEK Aurora is a 5-laser spectral cytometer that allows each fluorochrome to express themselves fully across the entire light spectrum. This permits the use of a wide array of fluorochrome combinations and delivers high-resolution data. The cytometer carries 5 solid state diode lasers (488nm Blue; 561nm Yellow; 638nm Red; 405nm Violet; 355 Ultraviolet), enabling more efficient spectrum capture in the 365-829 nm range. No filter changes are required for any fluorochrome excited by the 355nm, 405nm, 488nm, 561nm, 640nm lasers. The instrument has been shown to analyze up to 40 colors.
Click here for a set of CYTEK Aurora guides.
Click here for a table of tested fluorochromes for CYTEK Aurora.
Click here for a set of tables containing details about CYTEK Aurora’s lasers.
CytoFLEX S
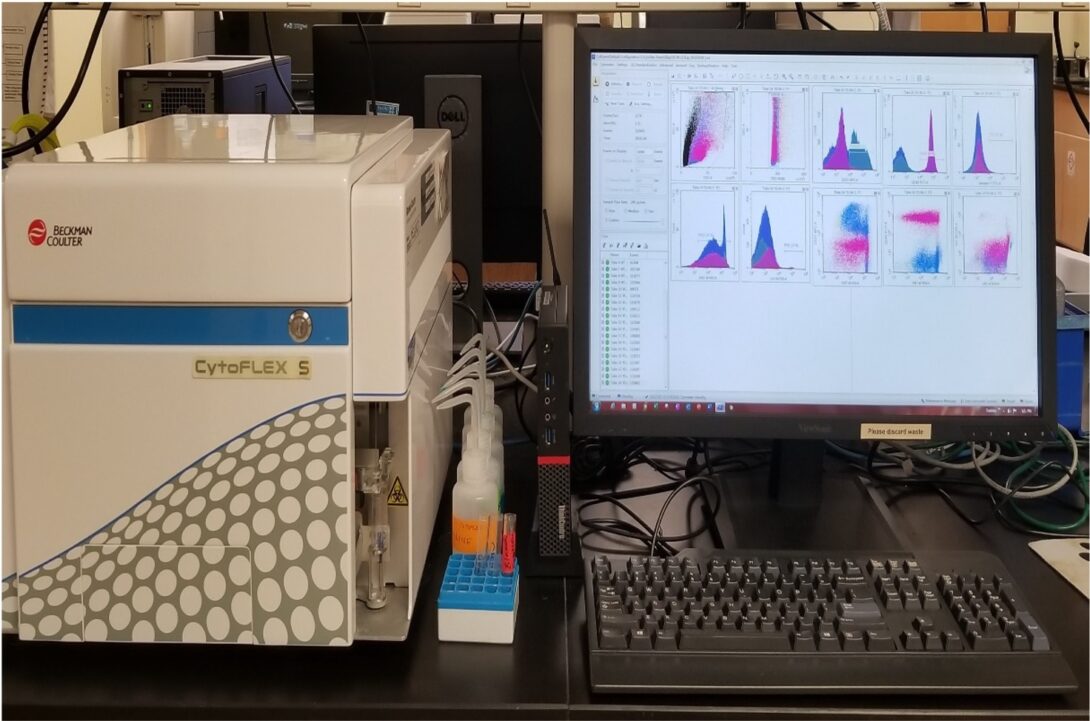
The CytoFLEX S is a benchtop flow cytometer that offers a semi-automatic single tube loading as well as a plate loader for 96-well plates and offers volumetric measurements with continuous flow. The cytometer carries 4 solid state diode lasers (488nm Blue, 561nm Yellow/Green, 638nm Red, 405nm Violet) and can analyze up to 13 colors (2B + 4Y/G + 3R + 4V).
Click here for a set of CytoFLEX guides.
Click here for a CytoFLEX S fluorochrome chart.
Click here for video tutorial.
Gallios
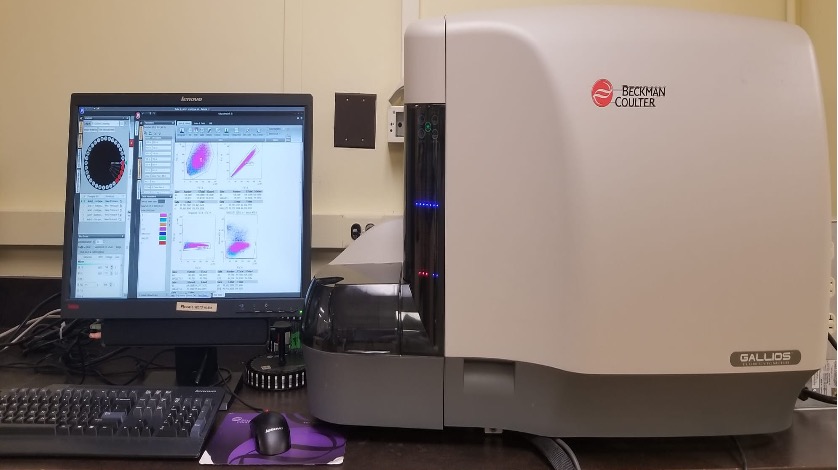
The Gallios flow cytometer provides efficient acquisition of excellent quality data from up to 10 colors with advanced optical design for enhanced sensitivity for multicolor assays. The multi-tube carousel loader provides quick and easy setup of automated walk-away processing of samples. The cytometer carries 3 solid state diode lasers (488nm Blue, 638nm Red & 405nm Violet) and can analyze up to 10 colors (5B + 3R + 2V). The Gallios also has a unique FSC detector that improves resolution of small particles.
Click here for a set of Gallios guides.
LSRFortessa with HTS

The BD LSRFortessa flow cytometer is configured with 4 lasers; blue, red, violet, and yellow-green, which enables the detection of up to 13 colors simultaneously. Arranged in octagon- and trigon-shaped optical pathways, their novel design efficiently maximizes signal detection and increases sensitivity and resolution. This allows researchers to identify cells, especially dim and rare cell populations, optimizing multicolor assays and panel design for superior results.
The LSRFortessa provides efficient acquisition of excellent quality data from up to 13 colors with advanced optical design for enhanced sensitivity for multicolor assays. The instrument is also equipped with a High Throughput System plate reader (BD HTS) that helps run 96-well and 384-well plates and provides for rapid, fully automated sample acquisition from microtiter plates, providing for easier processing of samples. The cytometer carries 4 solid state diode lasers (488nm Blue, 561nm Yellow 638nm Red & 405nm Violet) and can analyze up to 13 colors (2B + 5Y/G + 3R + 3V).
Click here for a set of LSRFortessa guides.
MoFlo Astrios

The MoFlo Astrios is a high-speed cell sorter with 5 lasers capable of 6-way sorting. It is equipped with 355nm, 405nm, 488nm, 561nm and 640nm lasers. The sorter is placed inside a Baker biosafety cabinet for sorting BSL-2 samples. Events can be sorted into different collection devices including microscope slides, micro tubes, 12×75 test tubes, and 15mL or 50mL Falcon tubes, as well as multi-well plates.
Reservations are made by calling 312-996-2775 (6-2775 from within UIC campus). You will need to provide information about the fluorochromes you are using for your assay, the number of cells, the kind of collection tubes you will collect sorted samples into, and any specific needs for your assay. You are strongly recommended to discuss your assay with the Director of the facility prior to preparing your experiments for sorting.
All sorts include a sort setup charge and a run time charge based on the scheduled and actual run. Cancelations must be made within 48 hours of run time to avoid incurring scheduled price charges. Final decisions to choose the appropriate cell sorter for the samples will rest with the Flow Cytometry Core staff based on requirement and availability.
Click here for a set of Sorter guides.
MoFlo Astrios EQ

The MoFlo Astrios EQ is a high-speed cell sorter with 4 lasers capable of 6-way sorting. It is equipped with 405nm, 488nm, 561nm and 640nm lasers. The sorter is placed inside a Baker biosafety cabinet for sorting BSL-2 samples. Events can be sorted into different collection devices including microscope slides, micro tubes, 12×75 test tubes, 15mL or 50mL Falcon tubes, as well as multi-well plates.
Reservations are made by calling 312-996-2775 (6-2775 from within UIC campus). You will need to provide information about the fluorochromes you are using for your assay, the number of cells, the kind of collection tubes you will collect sorted samples into, and any specific needs for your assay. You are strongly recommended to discuss your assay with the Director of the facility prior to preparing your experiments for sorting.
All sorts include a sort setup charge and a run time charge based on the scheduled and actual run. Cancelations must be made within 48 hours of run time to avoid incurring scheduled price charges. Final decisions to choose the appropriate cell sorter for the samples will rest with the Flow Cytometry Core staff based on requirement and availability.
Click here for a set of Sorter guides.
NanoSight NS300
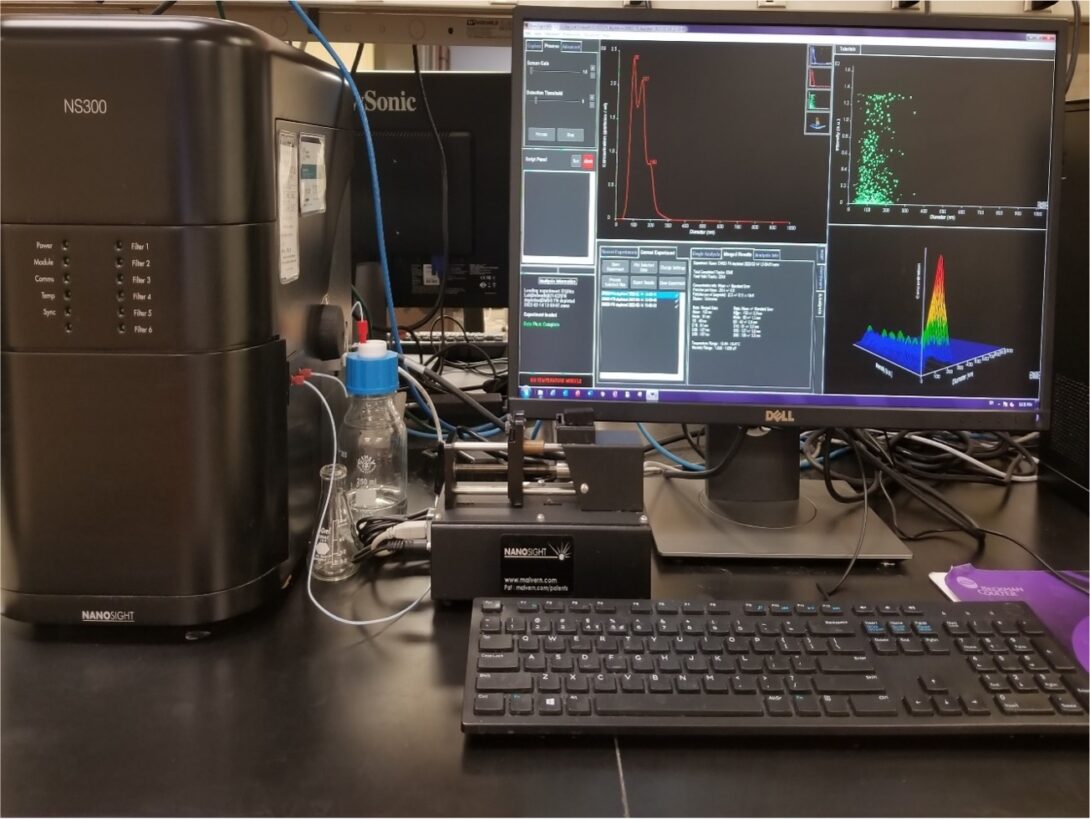
The Nanosight NS300 allows analysis of the size distribution and concentration of nanoparticles between 30nm to 2000nm in diameter.
Zetasizer

The Malvern Zetasizer Nano instrument is a particle analyzer that can measure three characteristics of particles from liquid samples: size (0.3nm to 10μm), molecular weight (3.8nm to 100μm), and zeta potential (342Da to 2x107Da).
These measurements can be taken in a wide range of concentrations, and measurement position is programmable.
Seahorse xFe96 Analyzer

The FCC provides training and access to the Seahorse XFe96 Analyzer. This instrument is an integrated system that that reports metabolic rates by measuring OCR and ECAR of live cells in a 96-well plate format. OCR and ECAR rates are indicators of mitochondrial respiration and glycolysis and thereby a reflection of cellular metabolic function.
MAGPIX

The MAGPIX is designed to analyze microsphere-based multiplex protein assays. This is an alternative to using ELISA assays to determine the concentration of proteins in solution, such as cell lysates. It can get results simultaneously for many proteins dissolved in a very small volume.
CyTOF Mass Cytometer

The CyTOF Mass Cytometer is a novel technology that combines some aspects of flow cytometry and with time-of-flight mass spectrometry. This technology utilizes metal-labeled antibody tags instead of fluorophores used in traditional flow cytometry, and as each elemental mass channel is distinct, requires minimal or no signal compensation. Importantly, the single cell resolution of flow cytometry is retained, and with the current setup, around 32 parameters can be measured using the CyTOF. The CyTOF thus permits the study of complex biological systems at the single-cell level.
SpectraMax M2 ELISA Reader

The SpectraMax M2 is a monochromator-based microplate reader that can collect absorbance and fluorescence data from samples, and can conduct endpoint, kinetic, well, and spectrum scanning.
The instrument has compatibility with 6-well to 384-well microplates and is compatible with absorbance and fluorescence cuvettes.
Absorbance wavelengths between 200-1000nm, excitation wavelengths between 250-850nm, and emission wavelengths from 360-850nm can be selected.
Cell Counter

The FCC houses the Vi-CELL XR Cell Viability Analyzer which provides an automated method to perform the Trypan Blue Dye Exclusion method, allowing users to load up to 9 samples at once for easy and automated cell analysis. Vi-CELL software offers pre-programmed and customizable analysis options for consistent and accurate analysis of simple cell systems or your own cell line.
Isospark

Isospark is an automated proteomics platform that provides information about single cell functional proteomics. The instrument is useful for determining functional immune landscaping, detecting immune biomarkers, and to study intracellular signaling Omics and signaling networks in cancer. The instrument can be used to detect biomarkers in biological fluids such as serum and plasma; cell supernatant and cell lysates and provides additional secretome information from single cells.
Work station
Workstation
The analysis workstation will be available for investigator who have run their samples in the FCC only and who have been trained in software analysis by FCC staff. will The workstation carries different analysis software to analyze flow cytometry data. Training will be provided to the investigators (see Analysis training under services) to use different software based on their needs.
The different software on the Workstation includes Summit, FlowJo, Kaluza, ModFit and WinMDI.